
Encyclopædia Britannica, inc. (2024, November 10). Periodic table. Encyclopædia Britannica. Britannica | Period Table.
Interactive Periodic Table of Elements: Elem.to

What is an element?
An element is a basic substance that can’t be broken down into simpler substances. Think of it like the simplest building block of matter. Each element is made up of just one kind of atom, like gold is made only of gold atoms, and oxygen is made of oxygen atoms. There are over a hundred different elements, and they each have their own properties—some are metals, some are gases, some react easily with other elements, and some don’t. When elements combine, they form all the different materials and compounds we see around us, like water, which is made from hydrogen and oxygen.
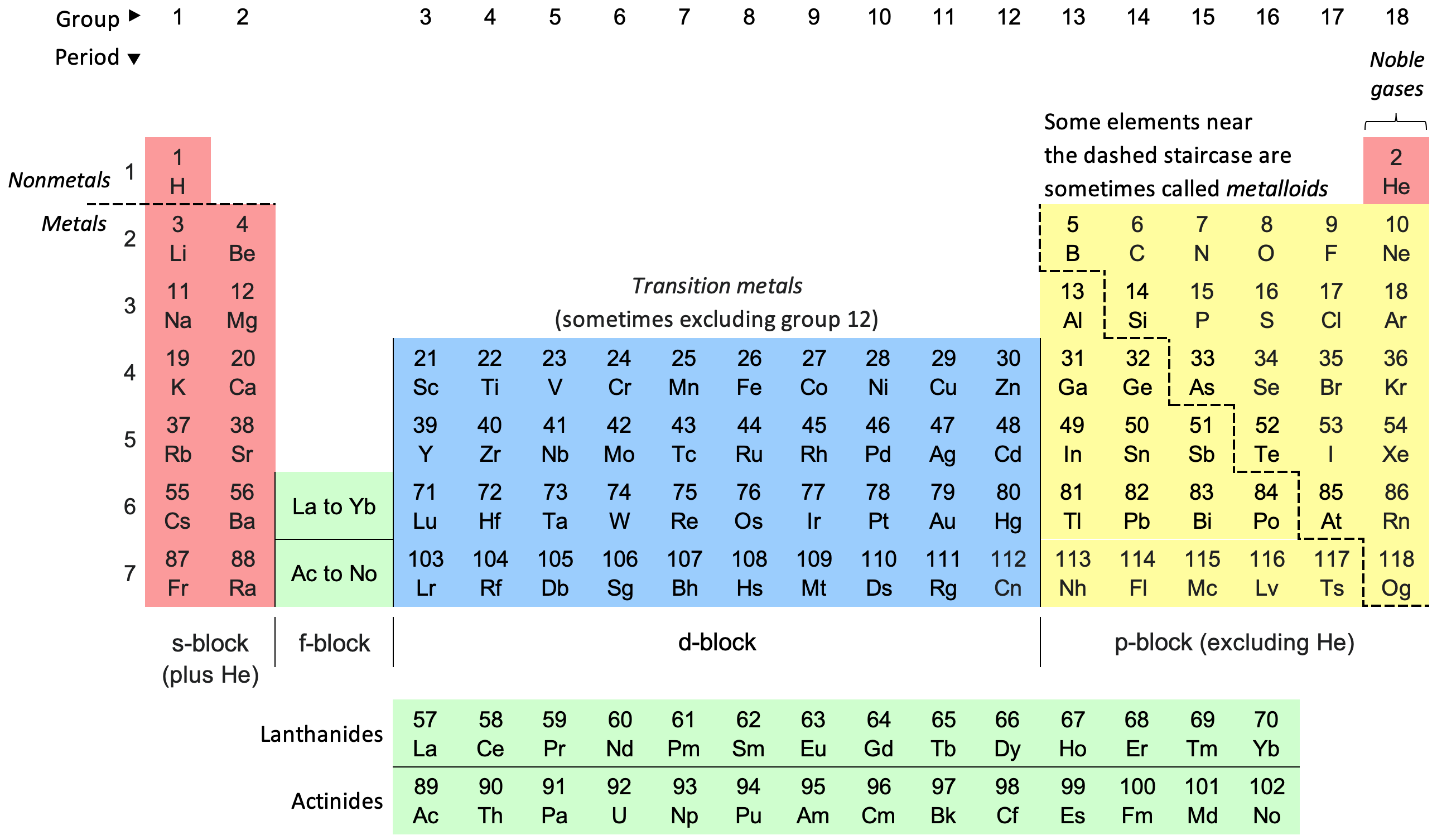
What is the periodic table of elements?
The periodic table of elements is a chart that organizes all known elements based on their properties. It’s like a map for understanding the building blocks of matter. Each element has a specific place in the table, arranged by its atomic number, which is the number of protons in its atoms. The table is divided into rows and columns that group elements with similar characteristics, like metals, non-metals, and gases. This organization helps scientists quickly see patterns in how elements behave, predict reactions, and understand how different elements interact to form everything around us.
How was it made?
The periodic table was created in 1869 by a Russian chemist named Dmitri Mendeleev. He arranged elements by their atomic weights and noticed that certain properties repeated in a pattern. Mendeleev left gaps for elements that hadn’t been discovered yet, predicting that they would be found to fit into those spaces, which later proved to be true. Over time, scientists refined the table to be organized by atomic number (the number of protons in an atom) instead of atomic weight, which made it even more accurate. The periodic table has since become a vital tool in chemistry, helping scientists understand elements and how they relate to one another.
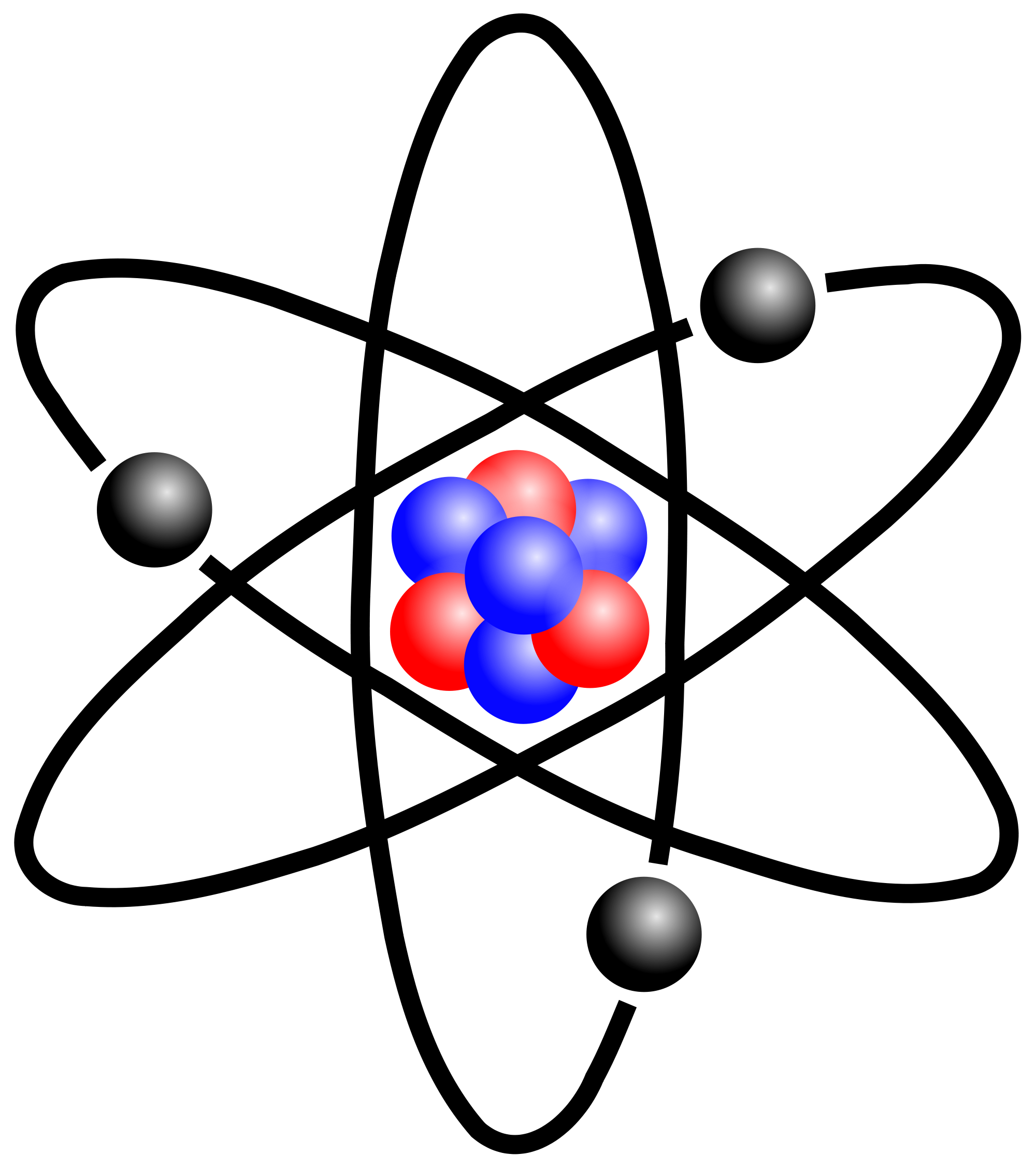
How is an element structured?
An element is structured at the atomic level, meaning it’s made up of tiny particles called atoms. Each atom of an element has three main parts: protons, neutrons, and electrons. The protons and neutrons are found in the center, or nucleus, of the atom, giving it most of its mass. The electrons move around the nucleus in regions called shells or orbitals. The number of protons in the nucleus defines what element it is—like hydrogen has one proton, while carbon has six. The arrangement of electrons around the nucleus influences how the element will interact with other elements, forming the basis for chemical reactions and bonds.
What is the "staircase"?
The staircase line on the periodic table helps show the difference between metals and nonmetals. It starts between boron (B) and aluminum (Al) and zigzags down to separate elements with metallic properties from those that don’t. Elements that are right next to this staircase are called metalloids. Metalloids have a mix of metal and nonmetal qualities. For example, they might look shiny like metals but be brittle and not conduct electricity as well. Common metalloids include boron (B), silicon (Si), and arsenic (As). On the left side of the staircase are metals, which are usually shiny, can conduct electricity, and are easy to shape. On the right side are nonmetals, which aren’t good conductors and can be solid, liquid, or gas.
Element Key
How are elements organized on the periodic table?
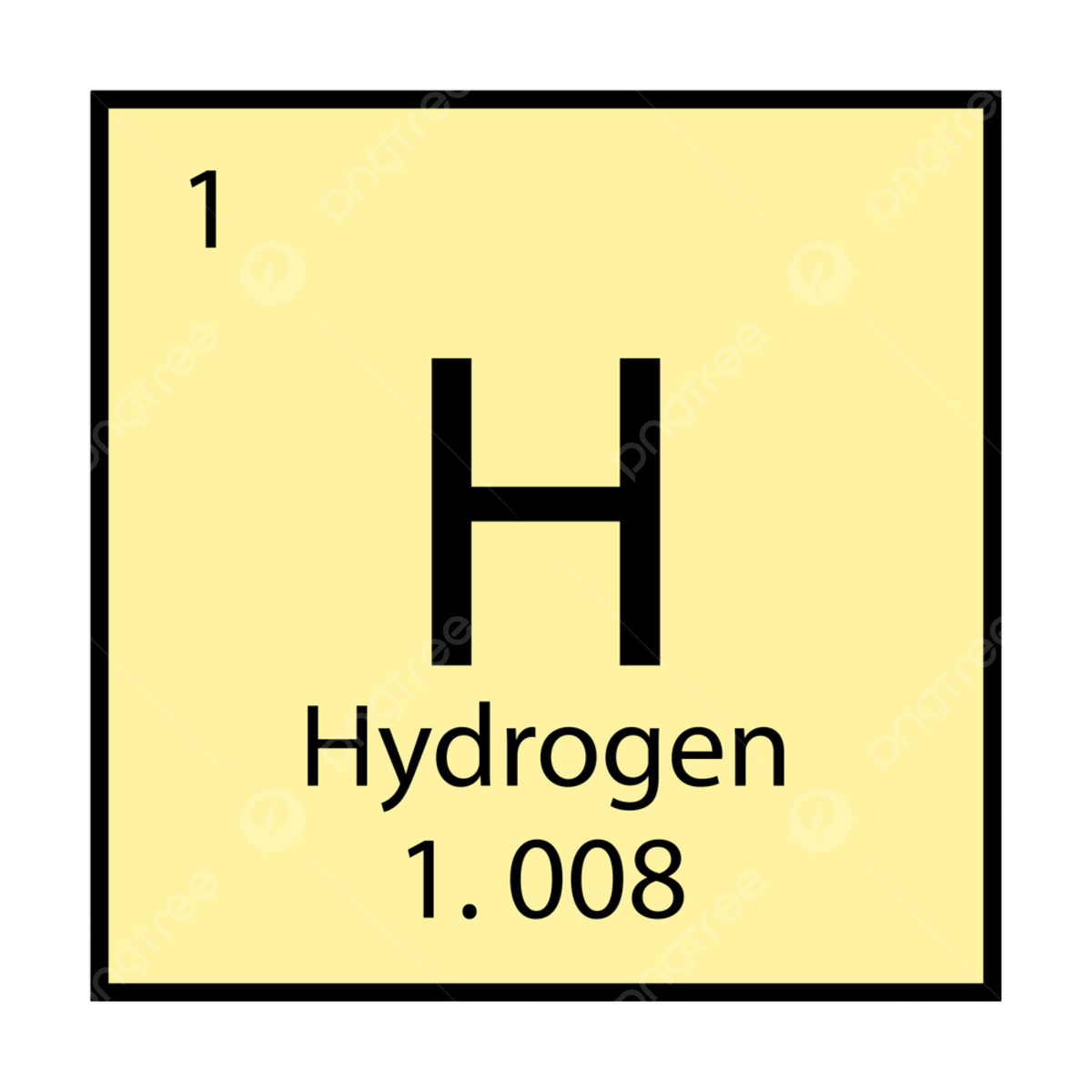
In the top left of the element you will see the number, "1". This number means that the element, Hydrogen, is the first element placed on the periodic table.
Next, in the middle of the element you can see the letter, "H". The "H" stands for Hydrogen, and "H" is it's chemical symbol.
Bellow you will see the name of the element, which in this case is Hydrogen.
And lastly, at the bottom of the element there is the atomic mass. The atomic mass is how much the atom ways at an atomic level. Hydrogen's atomic mass is 1.008.
What are periods?
Periods are the horizontal rows on the periodic table, where elements are arranged based on their increasing atomic number. Each period starts with an element that has a single electron in its outermost shell and ends with a noble gas, which has a full outer shell, meaning it's stable and less likely to react. As you move across a period from left to right, elements gradually change in properties, becoming less metallic and more nonmetallic. The elements in each period don’t share similar characteristics like element families, but they do have the same number of electron shells, which influences their size and how they interact with other elements. Studying periods helps us see trends in element properties, such as changes in reactivity and atomic size across each row.
What are families?
Element families are groups of elements in the periodic table that share similar chemical and physical properties due to having the same number of electrons in their outermost shell. These families, also called groups, are arranged in vertical columns on the periodic table and include groups like alkali metals, halogens, and noble gases. Elements in the same family typically behave in similar ways during chemical reactions. For example, alkali metals like lithium, sodium, and potassium are all very reactive and easily lose one electron, while noble gases like helium and neon are generally nonreactive because they have full outer electron shells. Understanding these families helps scientists predict how different elements will interact in compounds and reactions.

Alkali Metals
Alkali metals consist of the elements in Group I (expect for Hydrogen) and they are all elements that have only have one valence electron. They are highly reactive and can burst into flames when exposed to air. This is why alkali metals combine with other elements in compounds. They react with water quickly and must be stored in oil. Francium is the most reactive alkali metal, located in the seventh row. The reactivity decreases up the group, making lithium the least reactive element. Physically, alkali metals are shiny and white with low melting and boiling points.

Alkali Earth Metals
Alkaline earth metals are a group of elements in the second column of the periodic table, including beryllium, magnesium, calcium, strontium, barium, and radium. These metals have two electrons in their outermost shell, which makes them fairly reactive, though not as reactive as the alkali metals in the first column. Because they tend to lose these two electrons in chemical reactions, they often form compounds with a +2 charge. Alkaline earth metals are typically shiny, silvery-white, and good conductors of electricity, and they are commonly found in minerals and the earth’s crust. Their properties and reactivity make them important in applications like construction materials, fireworks, and even our bones, as calcium is a key component in human bone structure.
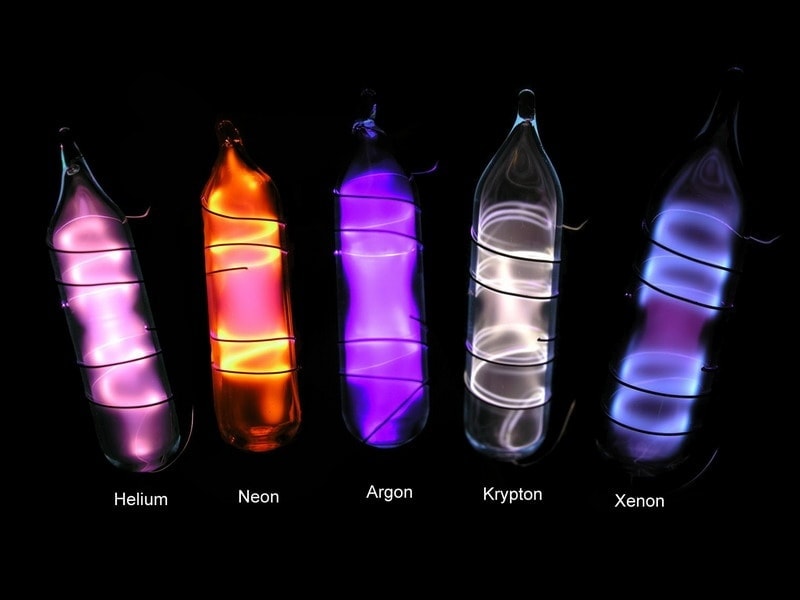
Noble Gases
Noble gases are a group of elements in the far-right column of the periodic table, including helium, neon, argon, krypton, xenon, and radon. These gases are unique because they have full outer electron shells, which makes them extremely stable and mostly unreactive with other elements. Due to their lack of reactivity, noble gases are often found in their pure form in nature and don’t easily form compounds. They are all colorless, odorless, and non-flammable under standard conditions, and each has distinct uses—helium, for example, is used in balloons because it’s lighter than air, and argon is used in light bulbs to prevent the filament from corroding. The stability and unique properties of noble gases make them valuable in many scientific and industrial applications.

Halogens
Halogens are a group of highly reactive elements in the second-to-last column of the periodic table, including fluorine, chlorine, bromine, iodine, and astatine. These elements have seven electrons in their outer shell, which makes them eager to gain one more electron to achieve a full, stable shell. This high reactivity means that halogens often form compounds, especially with metals, creating salts like sodium chloride (table salt). Halogens are found in various physical states at room temperature—fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid. Their reactivity and ability to form compounds easily make them useful in many areas, such as disinfectants, medicines, and even lighting.
Interactive Periodic Table of Elements: (click an element to learn more about it)
Quiz yourself!: 9 Question Quiz.
Share this website!
